
Minus K Educational Giveaway - November 2024
Single-molecule Microscopy Techniques and Negative-Stiffness Vibration Isolation Support DNA Superstructure Research at the University of Texas, Dallas
Imaging of samples using single-molecule microscopy is particularly challenged by Z-axis (vertical) noise, requiring a high-performance level of vibration isolation. Research into applications involving imaging and characterization of DNA and protein-DNA complexes, being performed at the University of Texas at Dallas, rely on atomic force microscopy supported by precision Negative-Stiffness vibration isolation.
DNA, or deoxyribonucleic acid, is the hereditary material in humans and almost all other organisms. Nearly every cell in a person's bodpersony has the same DNA, most of which is located in the cell nucleus. The DNA molecule is packaged into thread-like structures called chromosomes. Each chromosome is made up of DNA tightly coiled many times around proteins that support its structure. The information in DNA is stored as a code made up of four chemical bases: adenine, guanine, cytosine and thymine, which are present in pairs. Human DNA (i.e., the human genome) consists of about 3 billion DNA base pairs, and more than 99 percent of these base pairs are the same in all humans. The order, or sequence, of these DNA base pairs determines the information available for building and maintaining an organism.
DNA Superstructure Research at the University of Texas, Dallas
Research into the functions of DNA has had a significant impact on the fields of medicine, biotechnology, and the life sciences. One such group that has been conducting research into DNA is the Physical Genomics Laboratory in theDepartment of Bioengineering, University of Texas at Dallas. Under the direction of Dr. Stephen Levene, Professor of Bioengineering, research into the characterization of biological macromolecules of protein-DNA complexes (DNA superstructures) has been ongoing for a number of years. Levene’s research on the flexibility and folding of DNA, mediated by protein-DNA interactions, has led to valuable insights into the physics and organizations of genomes, as well as gene regulation and genetic recombination. His work has been supported by the American Cancer Society, Department of Defense, National Institutes of Health, National Science Foundation and the Texas Higher Education Coordinating Board.
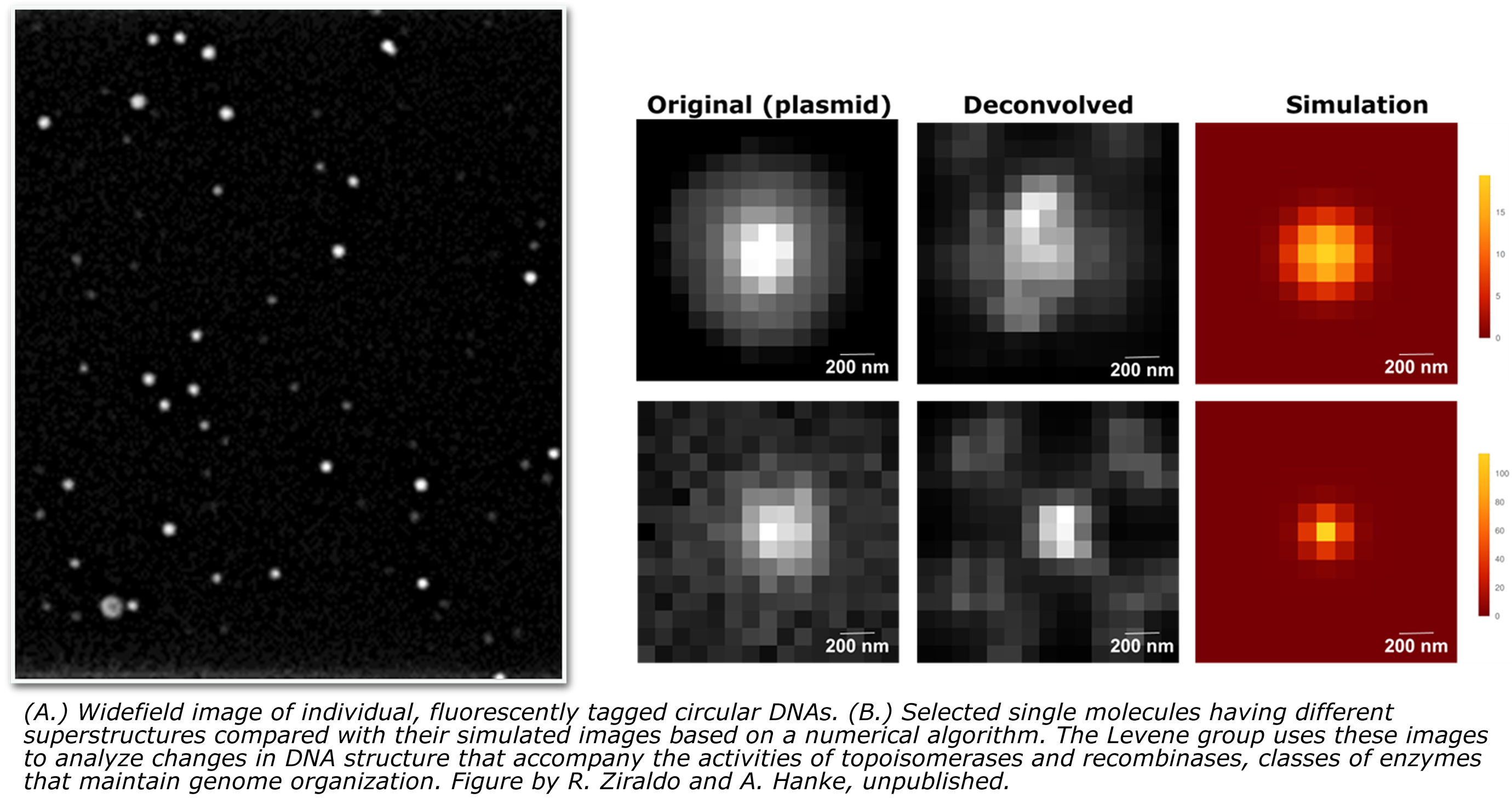
“I’ve been working on problems having to do with the interactions of proteins that organize DNA into tertiary structures that are biologically important,” said Dr. Levene. “We are interested in questions that have to do with DNA superstructures, higher-order structures that organize the human genome.”
“It is not unusual in the genome of many organisms for genes to be regulated by sequences of DNA that are many thousands of base pairs away,” continued Dr. Levene. “In fact, chromosomes are probably organized into functional units where regulatory elements occupy the same domain and genes that they regulate. By regulatory elements, I mean sequences of DNA where proteins bind, and turn on or turn off the genes that are under the control of those sequences.”
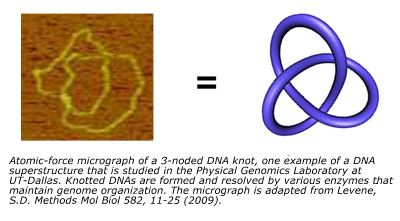 "So to that end, for most of my career I have been interested in what governs those interactions in physical terms, and more recently, how we can harness our understanding of those interactions to effect regulation of certain genes and to develop new strategies for diagnostics and therapeutics, added Dr. Levene." Dr. Levene's team performs experiments in both ensemble or bulk measurements (making measurements on huge numbers of molecules), as well as in living cells and on single molecules. Their group can thus infer the properties of these molecular species either indirectly or more directly visualize them using tools that resolve optical images of fluorescently labeled DNA molecules or visualize DNA structures by using atomic-force microscopy. There are advantages and disadvantages to both methodologies, so they pursue both of these scales simultaneously.
"So to that end, for most of my career I have been interested in what governs those interactions in physical terms, and more recently, how we can harness our understanding of those interactions to effect regulation of certain genes and to develop new strategies for diagnostics and therapeutics, added Dr. Levene." Dr. Levene's team performs experiments in both ensemble or bulk measurements (making measurements on huge numbers of molecules), as well as in living cells and on single molecules. Their group can thus infer the properties of these molecular species either indirectly or more directly visualize them using tools that resolve optical images of fluorescently labeled DNA molecules or visualize DNA structures by using atomic-force microscopy. There are advantages and disadvantages to both methodologies, so they pursue both of these scales simultaneously.
A unique aspect of Dr. Levene's research is that it combines its experimental research results with advanced computer models and other types of calculations to address very challenging physics issues.
"We study very complex problems - such as how DNA organizes under the constraints of environmental factors - requiring that we collect data on three-dimensional structure-dependent parameters," explained Dr. Levene. "For example, with coiling of DNA, which occurs spontaneously, called supercoiling - to interpret this, good theory is needed, which involves quite sophisticated physics, and requires using computer-simulation-based tools and or other computational-modeling approaches. Our group is probably regarded as one of the leading labs in terms of DNA tertiary structure. But this is largely because of a strong computational component through our research, which I think is unique."

AFM Vibration Problem
Although the UT-Dallas Bioengineering department has a number of AFMs for teaching purposes, the Physical Genomics Lab has a Bruker NanoScope VIII atomic-force microscope for research, which it uses along with associated instrumentation for liquid-cell or cryostatic imaging, single-molecule force spectroscopy, and nanoindentation studies. Most of the AFM's applications involve imaging and characterization of biological macromolecules (protein-DNA complexes) and composite bio-nanomaterials.
"We were using the vibration isolation systems that came with our AFMs," continued Dr. Levene. "Basically, a brick-like paving tile, suspended on bungee cords hooked to a tripod. This is the standard isolation system that comes with a $150,000 AFM. But this isolation method is rather inconvenient to work with and its performance can be inconsistent. We wanted to upgrade our isolation systems, especially since the buildings we are housed in on campus are exceptionally prone to vibration problems - in some locations the broad-band ambient vibrational noise is approximately 100 times the theoretical vertical resolution of the AFM instruments. None of our buildings are really quiet enough for standard methods of isolation, like bungee cords or air tables, to adequately isolate vibrations for these sensitive instruments."
Isolating a laboratory's sensitive instrumentation against low-frequency vibrations has become increasingly more vital to maintaining imaging quality and data integrity.
Low-hertz vibrations can be caused by a multitude of factors. Every structure is transmitting noise. Within the building itself, the heating and ventilation system, fans, pumps and elevators are just some of the mechanical devices that create vibration. Movement of people within the building is another source of vibrations. Depending on how far away the lab equipment is from these vibration sources, and where in the structure the equipment is located, whether on the third floor or in the basement, for example, will determine how strongly the instrumentation will be influenced. External to the building, the lab's research can be influenced by vibrations from vehicle movement, nearby construction, noise from aircraft, and even wind and other weather conditions can cause movement of the structure.
Negative-Stiffness Vibration Isolation
Over the past ten years, Dr. Levene's lab has been an avid user of Negative-Stiffness vibration isolators. It has installed two Negative-Stiffness isolation systems for laser-based instruments, and four isolators for teaching-lab AFM instruments. These instruments are distinct from the NanoScope VIII AFM, which is being used as a research instrument. A Negative-Stiffness isolation unit was also selected for use with the Physical Genomics Laboratory's new Leica Thunder fluorescence microscope system.

Physical Genomics Laboratory's Leica Thunder fluorescence microscope system on Minus K MK26 vibration isolation workstation
Developed by Minus K Technology (www.minusk.com), Negative-Stiffness isolators employ a unique and completely mechanical concept in low-frequency vibration isolation. They do not require electricity or compressed air. There are no motors, pumps or chambers, and no maintenance because there is nothing to wear out. They operate purely in a passive mechanical mode. Because of their very high vibration isolation efficiencies, particularly in the low frequencies, Negative-Stiffness vibration isolation systems enable vibration-sensitive instruments, such as AFMs, to operate in severe low-vibration environments that would not be practical with top-performance air tables and other vibration-mitigation technologies.
"Vertical-motion isolation is provided by a stiff spring that supports a weight load, combined with a Negative-Stiffness mechanism," said Erik Runge, Vice President of Engineering at Minus K Technology. "The net vertical stiffness is made very low without affecting the static load-supporting capability of the spring. Beam columns connected in series with the vertical-motion isolator provide horizontal-motion isolation. A beam column behaves as a spring combined with a negative-stiffness mechanism. The result is a compact passive isolator capable of low vertical and horizontal natural frequencies and high internal structural frequencies."
Negative-Stiffness isolators achieve a high level of isolation in multiple directions. They have the flexibility of custom tailoring resonant frequencies vertically to 0.5 Hz* and horizontally to 1.5 Hz (with some versions as low as 0.5 Hz horizontally).
(*Note that for an isolation system with a 0.5 Hz natural frequency, isolation begins at 0.7 Hz and improves with increase in the vibration frequency. The natural frequency is more commonly used to describe the system performance.)
These isolators resonate at 0.5 Hz. At this frequency there is almost no energy present. It would be very unusual to find a significant vibration at 0.5 Hz. Vibrations with frequencies above 0.7 Hz (where negative-stiffness isolators begin isolating) are rapidly attenuated with increases in frequency. When adjusted to 0.5 Hz, Negative-Stiffness isolators achieve approximately 93 percent isolation efficiency at 2 Hz; 99 percent at 5 Hz; and 99.7 percent at 10 Hz.
Performance
“Any researcher who uses atomic force microscopy appreciates the importance of vibration isolation,” said Dr. Levene. “The Bruker NanoScope AFM is located on the basement level of our Bioengineering and Sciences Building. This is a good location, but there’s nevertheless no guarantee of good vibrational-noise isolation given the proximity of the instrument to other vibration-generating mechanical equipment. With the Negative-Stiffness isolator, we have seen major benefits in terms of noise reduction, along with greater payload flexibility.”
Noise levels were quantified by measuring vertical-displacement noise with AFM probes that are in stationary contact with an atomically flat surface (cleaved highly-oriented pyrolytic graphite, HOPG). The time series of AFM probe vertical displacement was subjected to FFT (fast fourier transform) analysis to compare noise spectra using both old and new isolation systems.
“We realized we could not perform the DNA superstructure research we needed with the Bruker NanoScope AFM without high-performance isolation,” added Dr. Levene. “Negative-Stiffness vibration isolation, with performance particularly in the low-scale hertz band, has enabled our research.”
About Dr. Stephen Levene, Professor of Bioengineering & Distinguished Chair, Department of Biological Sciences, University of Texas at Dallas
Dr. Stephen Levene applies chemistry, mathematics, and molecular and cell biology expertise to applications in biotechnology. His research focuses on protein-DNA interactions in site-specific recombination, gene regulation, and other systems relevant to mechanisms of cellular programming.
Levene’s research on the flexibility and folding of DNA, mediated by protein-DNA interactions, has led to valuable insights into the physics and organizations of genomes, as well as gene regulation and genetic recombination. His work has been supported by the American Cancer Society, Department of Defense, National Institutes of Health, National Science Foundation and the Texas Higher Education Coordinating Board.
Dr. Levene holds a PhD in chemistry from Yale University. His work there led to the discovery of sequence-dependent bending in DNA, work published in the Proceedings of the National Academy of Sciences. He holds a bachelor’s degree in chemistry from Columbia University.
He has served as an editorial board member of the Journal of Experimental Biology and Medicine, and is an associate editor of BMC Biochemistry.
For more information, contact Stephen Levene, Ph.D., Department of Biological Sciences, University of Texas at Dallas, BSB 12, 800 West Campbell Road, Richardson, Texas 75080; Telephone 972-883-2503; email stephen.levene@utdallas.edu; www.utdallas.edu.
|